Does Benzene Exhibit Dipole Dipole Interactions
In short it is another type of intermolecular electrostatic interaction that occurs between a hydrogen atom bonded to an. Formaldehyde is a one-carbon aldehyde.

Schematization Of Dipole Dipole Energy Transfer Fret A From A Download Scientific Diagram
No although the c - cl bond is a polar bond due to the difference in paulings electronegativity values the actual molecule is.
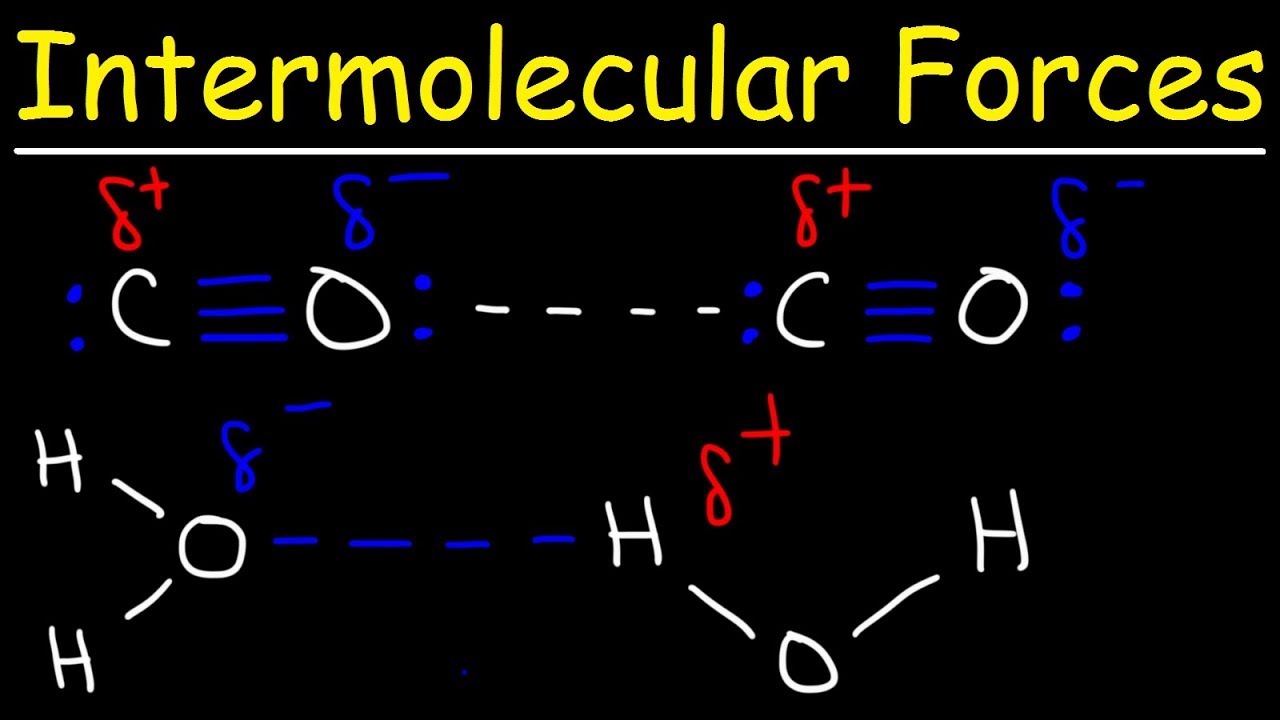
. Non-polar molecules do not exhibit dipole-dipole interactions. Feb 23 2017. London Dispersion Forces LDF.
Ion-Dipole Forces are involved in solutions where an ionic compound is dissolved into a polar solvent like that of a solution of table salt NaCl in water. This is due to an asymmetrical shape due to lone pairs of electrons around the central atom andor due to the presence of polar-covalent intra-molecular bonds electronegativity difference between the two atoms of 05. Strongest van der waals forces hydrogen bonding covalent bonding ionic bonding Submit Request Answer Answer A.
Benzene C6H6 310 Ethanol C2H5OH 393 Water H2O 408 Methane CH4 92 A Argon. An electric monopole is a single charge while a dipole is two opposite charges closely spaced to each other. The dipole-dipole interaction consists of the strongest intermolecular forces.
Look at the table in problem 20 benzene which only has London dispersion forces has a higher boiling point than acetone which has dipole-dipole and London dispersion forces. Exhibit Dipole-Dipole Interaction Does not. Th View the full answer.
Permanent dipole-permanent dipole interactions Polar molecules have an asymmetrical electron cloudcharge distribution. Just look at the trend for hexane nonpolar London dispersion interactions only 3-hexanone dipole-dipole interactions and 3-hexanol hydrogen bonding. There is overlap between the strengths.
Isobutylene on the other hand is a nonpolar molecule lacking dipole-dipole interactions since it only consists of C-C and C-H bonds. Note these must be for solutions and not pure substances as they involve two different species an ion and a polar molecule. Which interactions and processes contribute to the dissolution of ionic compounds in water.
Dipole-Dipole Interactions An example of a polar molecule would be CH3Cl or chloromethane. Exhibit Dispersion forces Does not exhibit Dispersion Forces BromobenzeneChlorobenzenePhenol Benzene B. As you would expect the strength of intermolecular hydrogen bonding and dipole-dipole interactions is reflected in higher boiling points.
Which one of the following exhibits dipole-dipole attraction between molecules. These interactions align the molecules to increase the attraction. Chloromethane is a carbon with three hydrogens and a chlorine attached to it.
It is not. Is exhibit pronoun or common noun. B Linear n-pentane molecules have a larger surface area and stronger intermolecular forces than spherical neopentane moleculesIn general however dipoledipole interactions in small polar molecules are significantly stronger than London dispersion forces so the former predominate.
When two of the dipole molecules interact with one another the negative portion of the polar molecule gets attracted to the positive part of the other molecule. Therefore the London dispersion forces must be greater than the dipole-dipole forces in this case. The benzene ring of chlorobenzene and bromobenzene exhibits this type of IMFA.
As it cooled the dipole-dipole interaction of water was stronger than the dipole-induced dipole or dipole-dipole interaction of benzene and water. The nature of inter-particle forces in benzene is A dipole-dipole interaction B dispersion force C ion-dipole interaction D H-bonding. These tend to happen only in the polar molecules like HCl.
Click hereto get an answer to your question Among the following mixtures dipole-dipole as the major interaction is present in 1 Benzene and CCIA 2 Benzene and CHOH 3 CHCOCH and CH CN 4 KCl and water. Benzoic acid has dipole-dipole and dipole-induced dipole interactions. This is present in all molecules and is the main force exhibited by non- polar molecules.
So there are electrostatic potential energy interaction terms for charge-dipole dipole-dipole dipole-quadrupole quadrupole-quadrupole etc. Check all that apply. What must the partial charge be on carbon.
Post boiling benzoic acid dissolved in water because the energy put into the system broke the intermolecular forces of benzoic acid making it miscible. The intermolecular interaction it exhibits is dipole-dipole interactions. Exhibit can be a noun or a verb.
Mainly hydrogen bonding but also dipole-dipole interactions E Mainly London-dispersion forces but also dipole-dipole interactions. These terms are important - the quadrupole-quadrupole interactions dictate the orientation of the benzene dimer and ceCO2 dimer in your example. Boiling Point and Hydrogen Bonding.
A XeF4 B AsH3 C CO2 D BCl3. Na H2On. Dipole-dipole interactions are electrostatic interactions between the permanent dipoles of different molecules.
Reset Help weakest Phenol exhibits intermolecular interaction among which is the covalent molecules. Yes in that order. Molecules that contain dipoles are called polar molecules.
The oxygen is more electronegative than the carbon so the oxygen holds the partial negative charge. Keeping this in consideration is pentane a dipole dipole. The carbon-hydrogen bonds are essentially non-polar but the carbon-chlorine bond is polar.
Does carbon tetrachloride exhibit dipole-dipole interactions. Click to see full answer. You can check the previous post for more details about the hydrogen bonding.
The art exhibit made him exhibit sympathetic feelings. But you dont need a spherically symmetric potential to end with a dipole of zero as in benzene.

Define Polar Nonpolar Dipole Dipole Forces Ion Dipole Forces Hydrogen Bonding And London Dispersion Forces Sublimation Condensation Evaporation Ppt Download
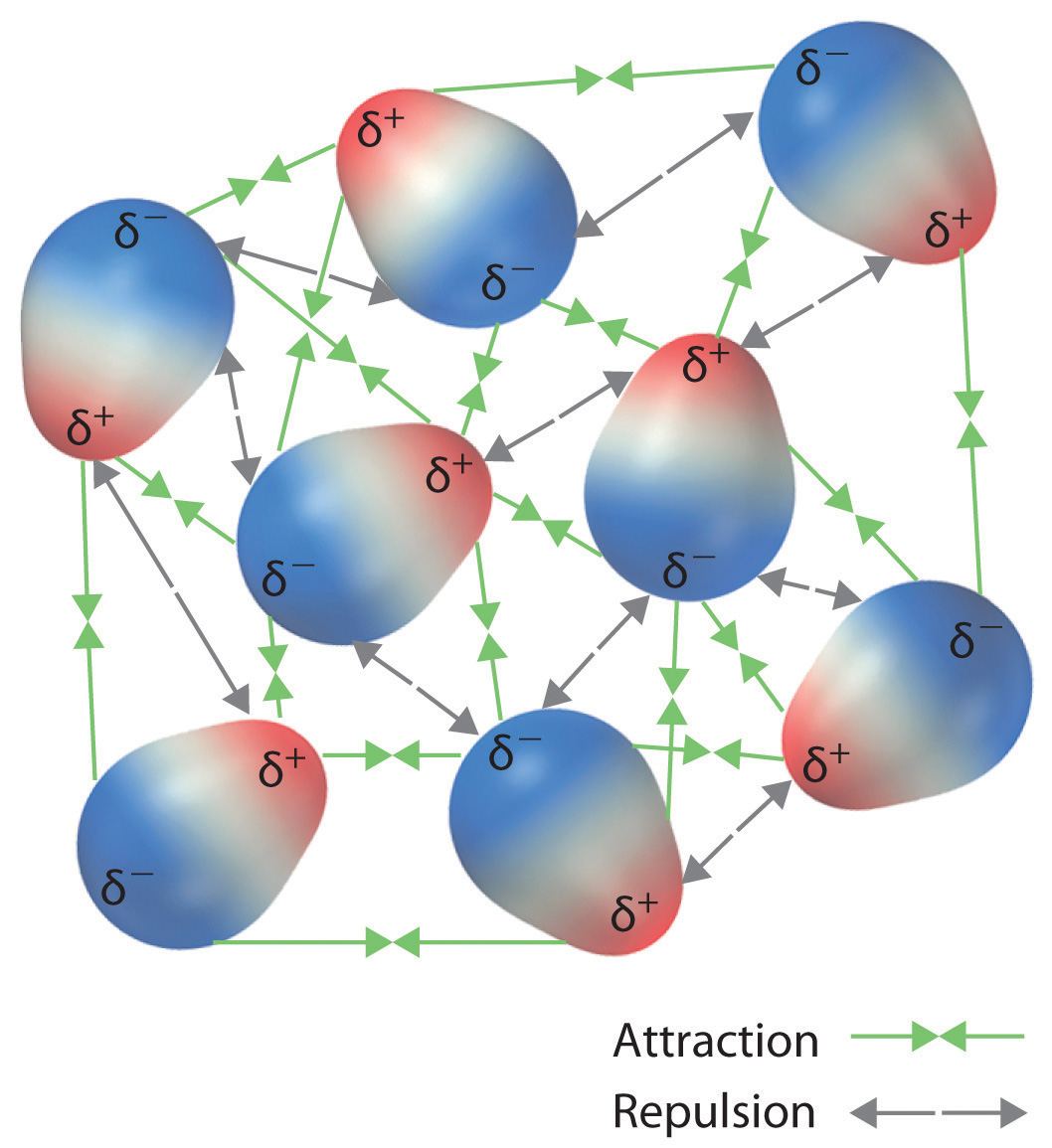
11 2 Intermolecular Forces Chemistry Libretexts

Intermolecular Forces Hydrogen Bonding Dipole Dipole Interactions Boiling Point Solubility Youtube

Intermolecular Forces Ppt Download
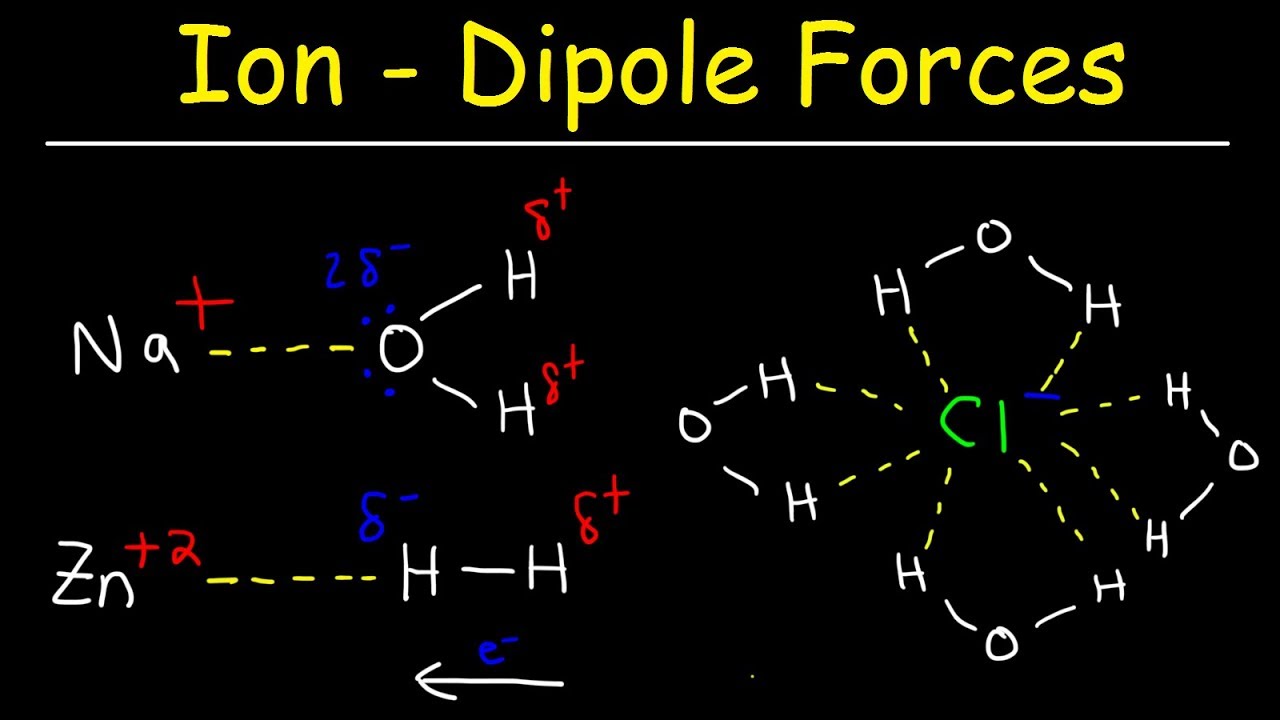
Ion Dipole Forces Ion Induced Dipole Interactions Chemistry Youtube
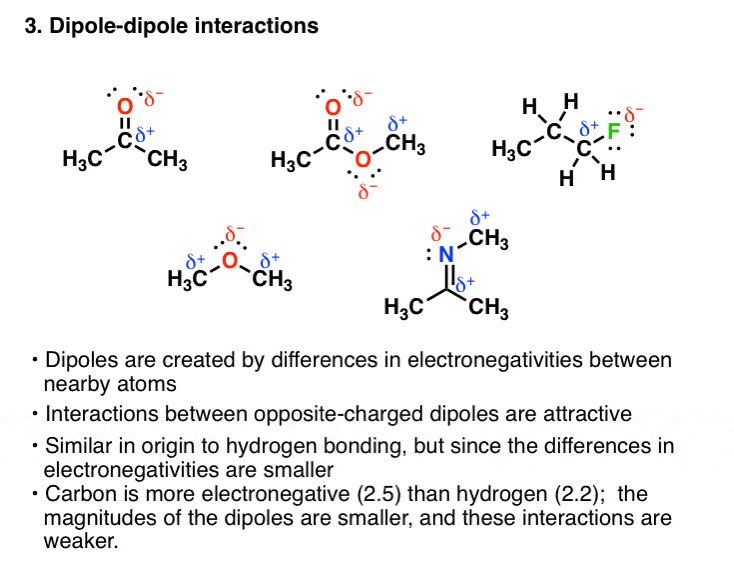
The Four Intermolecular Forces And How They Affect Boiling Points
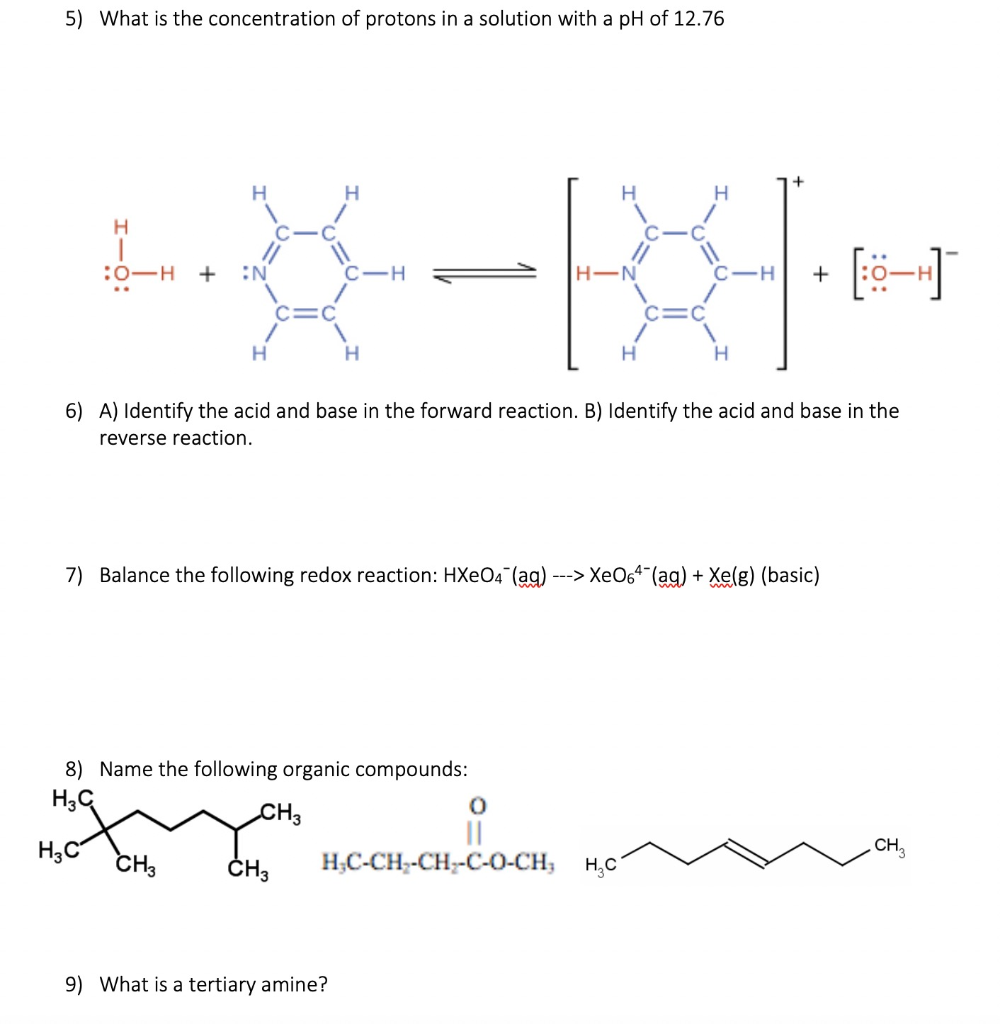
Solved 1 Intermolecular Forces Can Be Separated Into A Chegg Com
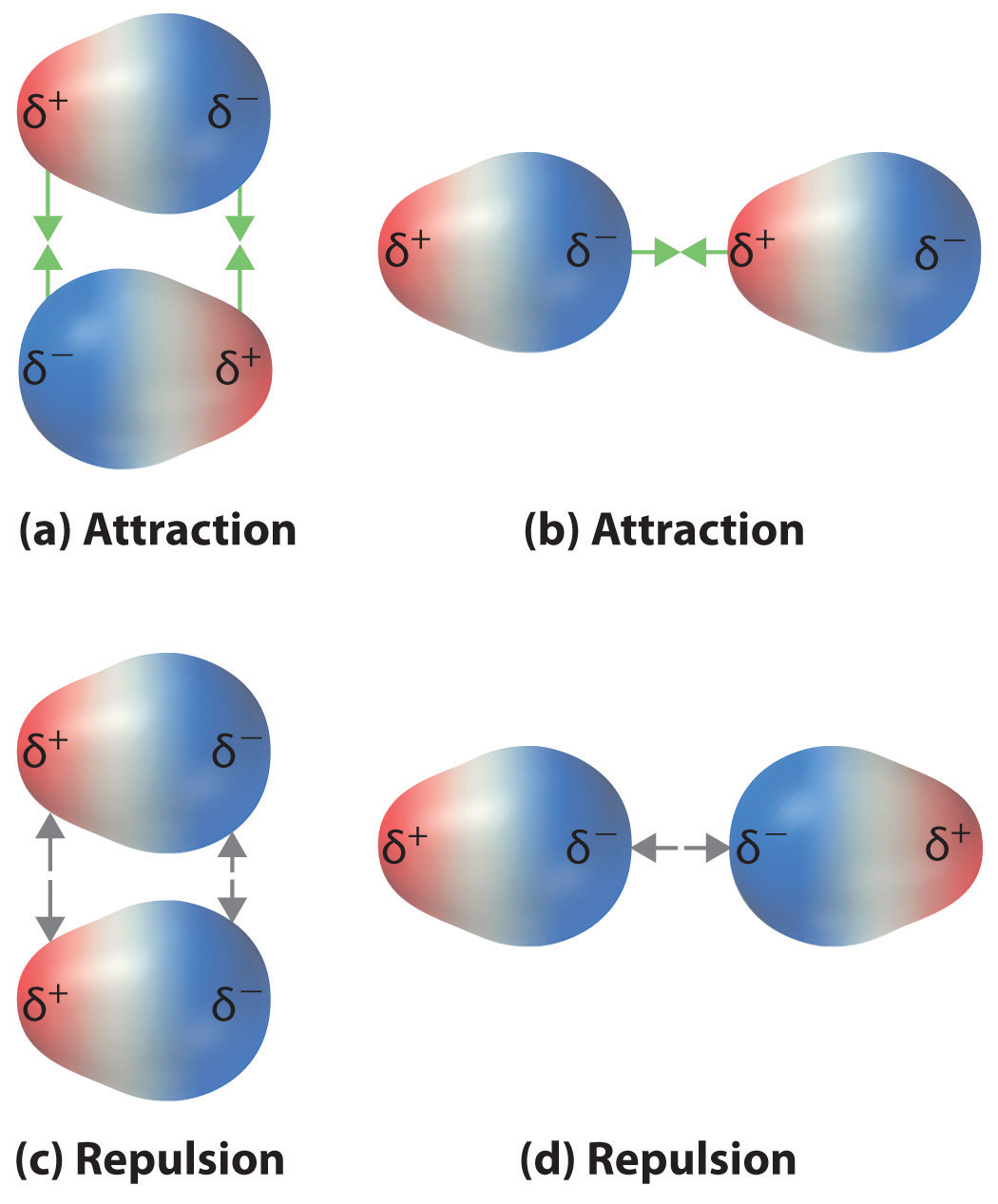
2 12 Noncovalent Interactions Between Molecules Chemistry Libretexts
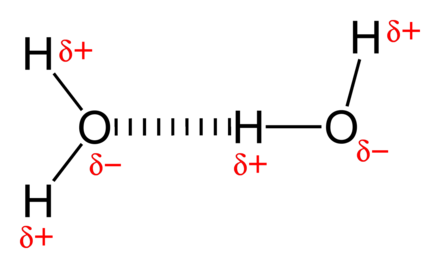
Non Covalent Interaction Wikiwand

Intermolecular Forces Ppt Download

3 Trends That Affect Boiling Points Master Organic Chemistry Organic Chemistry Chemistry High School Chemistry

Download Dipole Images For Free

Are Permanent Dipole Dipole Interactions Really Stronger Than The London Dispersion Force R Chemistry
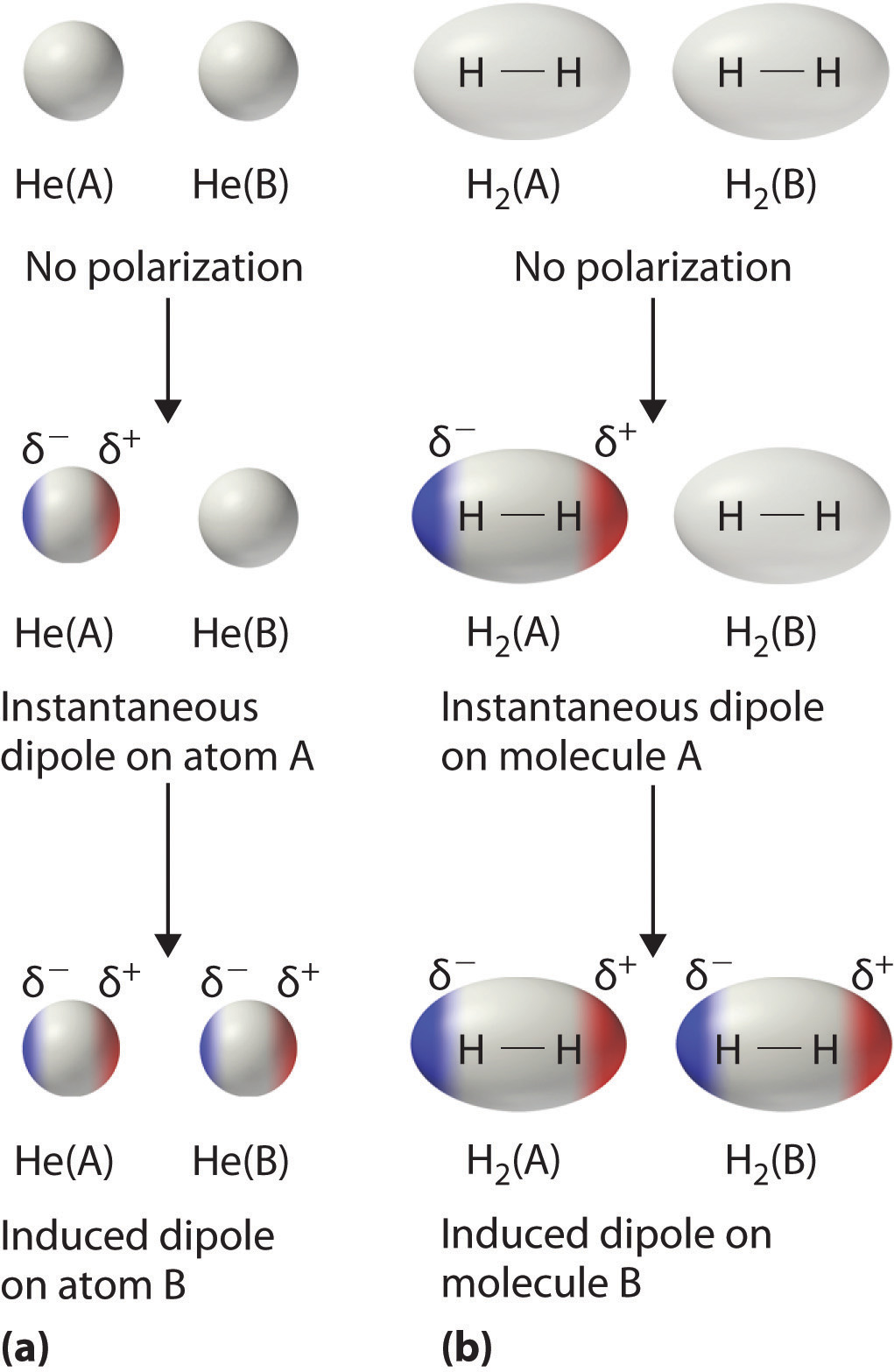
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts

Solved 1 Intermolecular Forces Can Be Separated Into A Chegg Com
Chem 245 Intermolecular Forces
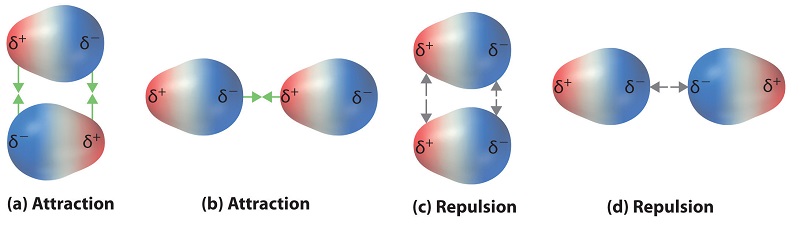
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
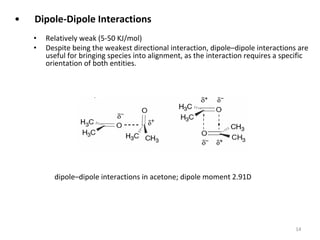

Comments
Post a Comment